| ABSTRACT
Despite extensive research on the pathogenesis of polycystic ovary syndrome (PCOS), there is still disagreement on the underlying mechanisms. The rat model for experimentally induced polycystic ovaries (PCO)--produced by a single injection of estradiol valerate--has similarities with human PCOS, and both are associated with hyperactivity in the sympathetic nervous system. Nerve growth factor (NGF) is known to serve as a neurotrophin for both the sympathetic and the sensory nervous systems and to enhance the activity of catecholaminergic and possibly other neuron types. Electro-acupuncture (EA) is known to reduce hyperactivity in the sympathetic nervous system. For these reasons, the model was used in the present study to investigate the effects of EA (12 treatments, approximately 25 min each, over 30 days) by analyzing NGF in the central nervous system and the endocrine organs, including the ovaries. The main findings in the present study were first, that significantly higher concentrations of NGF were found in the ovaries and the adrenal glands in the rats in the PCO model than in the control rats that were only injected with the vehicle (oil or NaCI). Second, that repeated EA treatments in PCO rats resulted in concentrations of NGF in the ovaries that were significantly lower than those in non-EA-treated PCO rats but were within a normal range that did not differ from those in the untreated oil and NaCI control groups. The results in the present study provide support for the theory that EA inhibits hyperactivity in the sympathetic nervous system.
adrenal, central nervous system, follicular development, hypothalamus, ovary, ovulation, pituitary, stress
INTRODUCTION
Polycystic ovary syndrome (PCOS), one of the most common causes of anovulation in women of reproductive age. is a complex endocrine and metabolic disorder [1]. Despite extensive research seeking the pathogenesis of PCOS, there is still disagreement on the underlying mechanisms. Different hypotheses of its pathophysiology have emerged, which indicates that the etiology is multifactorial and poorly understood.
Women with PCOS have an increased risk of endometrial cancer, hypertension, and type II diabetes, and they need some kind of long-standing treatment [2]. Traditional pharmacological treatment for ovulation induction is effective, but side effects such as superovulation are quite common. A previous clinical study on anovulatory women with PCOS showed that sensory stimulation (i.e., electro-acupuncture [EA]) affects endocrinological and neuroendocrinological parameters [3]. In addition, regular ovulations were induced in more than one-third of the women without negative side effects. These findings accord with previous reports [4-6] but do not enlighten underlying mechanisms. The mechanisms behind the beneficial effect of EA on PCOS in the human are difficult to study because tissue samples from the ovaries and the central nervous system (CNS) are for obvious reasons unobtainable. Studies on, for instance, neuropeptides in the gonads and the CNS would be possible to conduct in an animal model, provided that such a model exists. Experiments on normal cycling rats have shown that exogenous estradiol valerate (EV), a long-acting estrogen, causes acyclicity and the formation of polycystic ovaries (PCO) [7, 8]. The changes include atretic antral follicles, follicular cysts with a well-developed theca cell layer, a diminished granulosa cell compartment, and luteinized cysts [7, 8]. Furthermore, the rats exhibited alterations in basal and pulsatile LH and FSH concentrations, changes in the pituitary response to GnRH, degenerative changes in the hypothalamus, altered opioid inhibitory tone on GnRH release, and high estradiol levels with a persistent pattern of constant estrus as assessed by vaginal smear [9, 10]. In addition, EV-induced PCO is associated with an increase in peripheral sympathetic outflow, evidenced by an increase in the release of norepinephrine (NE), an increase in ovarian NE content, and a decrease in the number of ß-adrenergic receptors in the ovarian compartments receiving catecholaminergic innervation [9-11]. Even if it is not possible to reproduce human PCOS using a rat model, it may provide important leads because a single injection of EV induces an anovulatory state that shares many endocrinological and morphological characteristics of human PCOS [7-13]. Thus, comparisons between the rat PCO model and human PCOS must be interpreted with caution because rat PCO ovaries contain multiple follicular cysts, the structure of which does not replicate the follicular growth arrest found in human PCOS. Contrary to previously held notions, the granulosa cells in the follicles accumulating in the human ovary are not atretic. However, both human PCOS and EV-induced PCO in rats may be associated with hyperactivity in the sympathetic nervous system. According to one theory, elevated concentrations of neurotransmitters found in women with PCOS and anovulation may be associated with psychological stress and with hyperactivity in the sympathetic nervous system [3, 12, 13]. That superior ovarian nerve transection restores estrus cyclicity and ovulatory capacity in rats with EV-induced PCO further supports the theories of sympathetic hyperactivity [9]. Other evidence of neuronal involvement is that ovarian sympathetic innervation is under trophic control by nerve growth factor (NGF) [14]. This is also supported by the fact that the expression of the genes that encode NGF and one of its receptors, the low-affinity NGF-receptor, was dramatically increased in the ovary 30 days after EV injection [11]. Ovarian NGF is principally synthesized in the cells of the follicular wall [15], which is the site where the sympathetic neurons project to the ovaries [14]. The increase in the synthesis of NGF and its receptor that precedes the formation of cysts suggests that after PCO has been induced by EV injection, the neurons innervating the ovary are subjected to an enhanced neurotrophic influence that contributes to their hyperactivation and to the maintenance of an abnormally elevated catecholaminergic tone in ovarian steroid secretions [9-11].
Aim of the Study
Because NGF is known to serve as a neurotrophin for both the sympathetic and the sensory nervous systems and to enhance the activity of catecholaminergic and possibly other neuron types [9, 11, 14, 16-22], and because EA is known to reduce hyperactivity in the sympathetic nervous system [23-25], the experimentally induced PCO model was used to study the effects of EA by analyzing NGF in the CNS and the endocrine organs, including the ovaries.
The first part of the present study investigated dose-response--the discovery of the exact dose of EV needed to produce fully developed polycystic ovaries. The second part of this study investigated treatment with EA--what contribution NGF made to the etiology and maintenance of EV-induced PCO in rats and if and to what extent EA has an effect on NGF and ovarian morphology in experimentally induced PCO.
MATERIALS AND METHODS
Fifty-nine virgin adult cycling Sprague-Dawley rats (Möllegaard, Denmark) weighing 190-210 g and with regular 4-day estrous cycles were used. The rats were housed at 22°C, four to a cage, with free access to pelleted food and tap water and with a 12L:12D cycle for at least 1 wk before and throughout the experimental period. All rats received a single i.m. injection of either EV (Riedeldehaen, Germany), oil, or 0.15 M NaCI (Kabi Pharmacia AB, Sweden) and were anesthetized with enfluran (EFRANE, Abbott Scandinavia, Kista, Sweden) and killed by decapitation. The local Animal Ethics Committee at Göteborg University, Sweden approved the study.
Dose-Response
Twenty-seven rats were injected with one of two different doses of EV in an oil solution or with oil alone to ascertain the optimal dose for induction of PCO [8]. They were decapitated on three different occasions (15, 30, or 60 days after i.m. injection) to elucidate precisely when the ovaries display characteristic features of well-defined PCO [7, 8]. Nine rats received 2 mg EV in 0.2 ml oil/rat, nine rats 4 mg EV in 0.2 ml oil/rat, and nine rats 0.2 ml oil alone. Three rats per dose were killed on Day 15, three on Day 30, and three on Day 60.
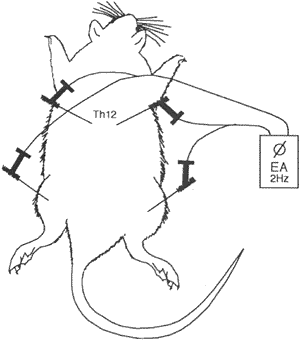
FIG. 1. Schematic drawing of the dorsal side of a rat and the placement of acupuncture needles. Two needles were placed bilaterally in the erector spinae muscle at the level of Th12 and two were placed in the quadriceps muscle bilaterally. The needles were then attached to an electrical stimulator for EA treatment.
Treatment with EA
The optimal dose (4 mg EV in 0.2 ml oil/rat) and timing (30 days after injection) were chosen for the experiments. In total, 32 rats took part. Eight rats in the EV control group and eight in the EA-treated EV group were injected i.m. with 4 mg EV in 0.2 ml oil/rat, eight rats in the oil control group with 0.2 ml oil, and eight rats in the NaCI control group with 0.2 ml 0.15 M NaCI. All 32 were decapitated on Day 30 after injection, that is, 1-2 days after the last EA treatment. All groups were anesthetized 12 times for about 25 min each time. Anesthesia was induced by inhalation of enfluran at 5.5-6.5 ml/h with an O2 and air flow of 0.25 L/min. The EA-treated EV group was subjected to 12 EA treatments every second or third day, beginning 2 days after the i.m. injection of EV. The stimulation points were bilateral in the quadriceps and erector spinae muscles at the level of thoracic (Th) 12 in the somatic segments according to the innervation of the ovaries (Th 12-lumbar [L]2, sacral [S]2-S4) (Fig. 1). The needles (Hegu; Hegu AB, Landsbro, Sweden) were inserted to depths of 0.5-0.8 cm and then bilaterally attached to an electrical stimulator (CEFAR ACU II, Cefar, Lund, Sweden) with a low burst frequency of 2 Hz. Individual pulses within the frequency were square wave pulses with alternating polarities and with a pulse duration of 0.2 msec, 80 pulses/sec. The intensity was adjusted so that local muscle contractions were seen to reflect the activation of muscle-nerve afferents (A delta fibers and possibly C fibers) [26, 27]. The location and type of stimulation were the same in all rats.
Nerve Growth Factor Measurements by Enzyme Immunoassay
In the second part of the study, after the rats were decapitated, the pituitary gland, the hypothalamus, the hippocampus, one ovary, and the adrenal glands were quickly removed and dissected on dry ice, weighed, and stored at -80°C until extraction. The samples were sonicated in extraction buffer (0.1% Triton X-100, 100 mM Tris-HCI, pH 7.2, 400 mM NaCI, 4 mM EDTA, 0.2 mM PMSF, 0.2 mM benzethonium chloride, 2 mM benzamidine, 40 U/ml aprotinin, 0.05% sodium azide, 2% BSA, and 0.5% gelatin; 1 ml/100 mg of tissue), followed by centrifugation at 10,000 x g for 30 min. The supernatants were used for the assay. The bioactive form of 2.5S NGF purified from mouse sub-maxillary glands and prepared in the laboratory at the Institute of Neurobiology (CNR) in Rome, Italy, according to the method of Bocchini and Angeletti [28] was used as a standard. The NGF was dissolved in extraction buffer and the standard curve was in a range of 31.25 pg ml (-1) and 1 ng ml (-1). An ELISA was performed as described by Weskamp and Otten [29] with a minor modification [30]. Specific NGF binding was assessed by use of monoclonal mouse anti-ß-2.5S NGF (Boehringer Mannheim GmbH, Mannheim, Germany) that reacts with both the 2.5S and the 7S biologically active forms of NGF. The absorbency of samples and standards was corrected for nonspecific binding (i.e., the absorbency in a well coated with purified mouse IgG). The NGF content in the samples was determined in relation to the NGF standard curve. Data were not corrected for recovery of NGF from samples, which was routinely 70-90%, and was accepted only when the values were >2 SD above the blank. With these criteria, the limit of sensitivity of NGF ELISA averaged 0.5 pg per assay.
Morphology
One ovary per rat was removed, cleaned of adherent connective fat tissue, and fixed in 4% formaldehyde buffer; sections were stained with hematoxylin-eosin, and a trained pathologist performed a quantitative analysis of the follicle population. If ovum degeneration or at least one pyknotic granulosa cell was seen, the follicle populations were classified as atretic, otherwise they were classified as healthy. Morphological characteristics of follicular atresia were, for instance, scattered pyknotic nuclei in the granulosa cell layer [31], detachment of the granulosa cell layer from the basement membrane [32], fragmentation of the basal lamina [33], and the presence of cell debris in the antrum of the follicle [34].
Statistical Analyses
Statistical analyses were carried out using the SPSS 8.0 software. The NGF concentrations in the pituitary gland, the hypothalamus, the hippocampus, the ovary, and the adrenal glands were analyzed and the groups compared using ANOVA followed by multiple comparison procedures (Bonferroni test). All results are presented as mean ± SEM. A P value less than 0.05 was considered significant. The 95% confidence interval (Cl) was given when P < 0.05.
RESULTS Ovarian Morphology--Dose-Response In the first part of the present study, dose-response, injection of 0.2 ml oil alone (control) was associated with a normal appearance of the ovaries and no differences were seen between rats sacrificed on Day 15, 30, or 60 (Fig. 2, a and b). No changes were seen in the ovaries of rats injected with 2 mg EV in 0.2 ml oil/rat and killed on Day 15. The ovaries of rats injected with the same dose of EV in oil exhibited small morphological changes resembling PCO when killed on Day 30 and 60 (Fig. 3, a and b). The ovaries of rats injected with a higher dose of EV (4 mg EV in 0.2 ml oil/rat) exhibited only small morphological changes on Day 15. Rats injected with the same dose of EV in oil and killed on Day 30 (Fig- 4, a-c) showed a progressive decrease in the number of primary and secondary follicles but it was on Day 60 (Fig- 5, a and b) that the true cystic follicles appeared and the well-defined PCO was fully developed in accordance with previous reports by Brawer et al. [8]. Ovarian Morphology--Treatment with EA In the second part of the present study, treatment with EA, all rats were killed at Day 30 after EV injection, i.e., before the appearance of cystic follicles. The ovaries in the EV control group (4 mg EV in 0-2 ml oil/rat) displayed the same morphological changes as previously shown in the dose-response section (see Fig- 4, a-c). The ovaries in the oil control group and the NaCI control group exhibited a typically normal appearance (see Fig- 2, a and b). No substantial morphological differences were found between the EA-treated, EV group, and the EV control group.
Nerve Growth Factor--Treatment with EA In the second part of the present study, treatment with EA, NGF measurements were made at Day 30 after EV injection. Means ± SEM for NGF (pg/g wet weight) in the hypothalamus, the pituitary gland, the hippocampus, the ovary, and the adrenal gland in all groups are presented in Table 1. Ovarian NGF concentrations were significantly higher in the EV control group compared to the oil control group (P < 0.001, CI = 178.7, 821.6) and the NaCl control group (P < 0.01, CI = 144.6, 787.5). The NGF concentrations in the ovary were significantly lower in the EA-treated, EV group compared to the EV control group (P < 0.05 Cl = 6.2, 614.9) and did not differ from the (Jil and the NaCI control groups) The NGF concentrations in the adrenal glands were significantly higher in the EV control group and the EA-treated. EV group compared to both the oil control group (P < 0.001, CI = 45.7, 169.3 and P < 0.01, CI = 38.5, 166.5) and the NaCI control group (P < 0.001, Cl = 21.9, 162.9 and P < 0.01, Cl = 15.0, 159.8). Weights of Ovaries and Adrenal Gland--Treatment with EA Means ± SEM for weights (mg) of the ovaries and the adrenal glands in all groups are presented in Table 2. Ovarian weights in the control EV group and in the EV-treated EV group were significantly lower compared to the oil control group (both P < 0.001) and the NaCI control group (both P < 0.001). 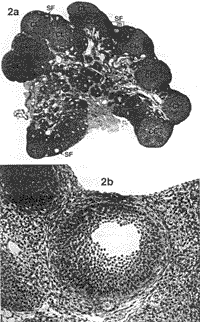 | 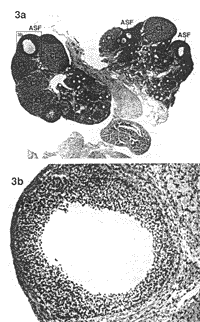 | | FIG. 2. a) Section of an ovary from a rat injected with 0.2 ml in oil and sacrificed on Day 30. In total, 11 corpora lutea (CL) marked with CL and three secondary follicles (SF) marked with SF are seen. One secondary follicle is framed (b). Magnification x2.5. Section stained with hematoxylin-eosin. b) Normal secondary follicle. Magnification x20. | FIG. 3. a) Section of an ovary from a rat injected with 2 mg EV in 0.2 ml oil and killed on Day 30. In total, six corpora lutea marked with CL and three atretic secondary follicles (ASF) marked with ASF are seen. The atretic secondary follicle is framed (b): Magnification x2.5: Section stained with hematoxylin-eosin. b) An atretic secondary follicle with granulosa cells showing signs of atresia and intact theca cells. Magnification x20. | 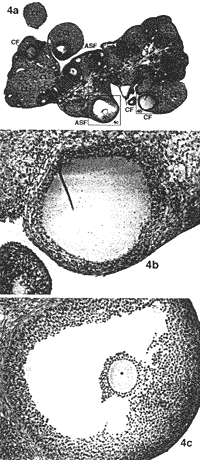 | 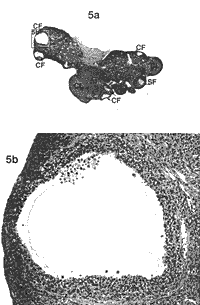 | | FIG. 4. a) Section of an ovary from a rat injected with 4 mg EV in 0.2 ml oil and sacrificed on Day 30. In total, seven corpora lutea marked with CL three cystic follicles (CF) marked with CF, and two atretic secondary follicles marked with ASF are seen. One cystic follicle (b) and one atretic secondary follicle are framed (c). Magnification x2 5: Section stained with hematoxylin-eosin. b) Cystic degenerating follicle showing a thin granulosa layer and debris in follicular fluid. Magnification x20. c) An atretic secondary follicle with detachment of the oocyte from the cumulus mass of pyknotic granulosa cells. Magnification x20. | FIG. 5. a) Section of an ovary from a rat injected with 4 mg EV in 0.2 ml oil and sacrificed on Day 60. In total, two corpora lutea marked with CL, five cystic follicles marked with CF and one secondary follicle marked with SF are seen. One cystic follicle is framed (b). Magnification x2.5 Section stained with hematoxylin-eosin. b) A cystic degenerating follicle showing a thin granulosa layer and debris in follicular fluid. Magnification x20. |
DISCUSSION The main findings in the present study are as, follows: First, PCO induced in rats by a single injection or EV results in significantly higher concentrations of NGF in the ovaries and the adrenal glands without any changes in the brain tissue when measured 30 days after EV injection. Second, repeated EA treatments with low frequency (2 Hz) significantly decrease the elevated NGF concentrations in the ovaries, to within a normal range, without affecting NGF concentrations in the adrenal glands or brain tissue when measured 30 days after EV injection. The histological examination of the ovaries in the first part of the present study, dose-response, revealed that the optimal dose of EV that caused typical PCO-like morphological changes was 4 mg and that PCO was fully developed at Day 60. This dose was twice that used by Brawer and coworkers [7, 8] to achieve full development of a well defined PCO in rats. The reason might be differences in the strain of rat and/or the estrogen preparation that was used. In addition, the ovarian weight in the two EV-injected groups was significantly lower compared to that in the vehicle-injected (oil and NaCI) control rats. The reduction in ovarian weight and size, as well, are in accordance with the findings of Brawer et al. [8]. The reduction in weight and size of the ovaries might be explained by a reduction in the number of corpora lutea. In the second part, treatment with EA, no substantial influence in ovarian morphology was seen at Day 30, after EV injection with the number and duration of the EA treatments used in this study. However, the main reason for beginning EA treatment as early as 2-3 days after EV injection and to decapitate at Day 30 after EV injection was to estimate whether EA could influence the increased ovarian NGF concentrations that have been shown to precede the development of morphological changes in rats with PCO [11]. It remains to be shown whether EA influences the ovarian morphology 60 days after EV injection. It would therefore be of interest to study the effects of EA after extended treatment periods. Such a study would provide a unique opportunity to collect experimental evidence of the effectiveness of EA in humans. In fact, we have observed that the multifollicular pattern characteristic of the ovarian morphology of women with PCOS and anovulation, as assessed by ultrasonography, began to disappear after they had received repeated EA treatments [3]. An involvement of the nervous system in the etiology and/or maintenance of PCOS is suggested by both clinical and experimental findings [9-13]. Clinical studies show that women with PCOS temporarily recover normal ovarian function after bilateral wedge resection or ovarian drilling that partially denervates the ovary [35, 36]. There is thus a possibility that the ovarian nerves are involved in the successful outcome of bilateral wedge resection and ovarian drilling.
Experimental observations in rats reveal that superior ovarian nerve transection in EV-induced PCO reduces the steroid response, increases ß-adrenoreceptor concentrations to more normal levels, and restores estrus cyclicity and ovulation [9]. These effects were linked to reduced activity in the ovarian sympathetic nerve fibers, indicating a peripheral neurogenic effect [9]. Sensory stimulation, i.e., EA, activates muscle-nerve afferents, mainly A-delta and possibly C fibers [23, 26, 27], that initiate a number of peripheral reactions at the spinal level and centrally in the brain. That EA may reduce hyperactivity in the ovarian peripheral sympathetic nerve fibers is in accordance with the theory that EA could modulate sensory, motor, and autonomic outflow at the segmental level [24]. In parallel, higher control systems are activated, resulting in the release of a number of neuropeptides, important in the modulation of central and segmental autonomic outflow, of the hypothalamic-pituitary-ovarian axis (HPO axis), and of the descending pain-inhibiting systems [23-25].
TABLE 1. Treatment with EA.
| | NGF concentration (pg/g)[a] | | | | | | EA-treated, EV
(n = 8) | EV control
(n=8) | Oil control
(n=8) | NaCI control
(n=8) | | | | Ovary | 647.8 ± 69.9[b] | 952.2 ± 95.1[d] | 452 ± 42.4 | 486 ± 90.0 | | Adrenal gland | 157.4 ± 11.8[c] | 162.4 ± 22.1[e] | 54.9 ± 9.7 | 70 ± 14.4 | | Pituitary gland | 63.3 ± 9.6 | 95.5 ± 15.5 | 122.6 ± 36.2 | 125.8 ± 26.0 | | Hypothalamus | 293.1 ± 26.8 | 293 ± 64.7 | 531.2 ± 155.7 | 315.6 ± 21.3 | | Hippocampus | 3412.4 ± 210.2 | 3589.6 ± 292.2 | 2837.9 ± 122.7 | 3166.0 ± 164.8 |
[b] P < 0.05, EA EV versus EV control.
[c] P < 0.001, EA EV versus oil control; and P < 0.01, EA EV versus NaCI control.
[d] P < 0.001, EV control versus oil control; and P < 0.01, EV control versus NaCI control.
[e] < 0.01, EV control versus oil control; and P < 0.001, EV control versus NaCI control.
TABLE 2. Treatment with EA.| | Weight (mg)[a] | | | | | | EA-treated, EV
(n = 8) | EV control
(n=8) | Oil control
(n=8) | NaCI control
(n=8) | | | | Ovary | 0.011 ± 0.0007[b] | 0.011 ± 0.0007[c] | 0.021 ± 0.001 | 0.02 ± 0.001 | | Adrenal gland | 0.018 ± 0.0008 | 0.016 ± 0.006 | 0.017 ± 0.001 | 0.018 ± 0.0011 |
[a] Weights of the ovary and the adrenal gland shown as mean ± SEM in the different groups: EA-treated, EV; EV control; oil control (0.2 ml); and NaCI control (0.2 ml 0.15 M). All EV injections were 4 mg EV in 0.2 ml oil/rat.
[b] P < 0.001, EA EV versus oil control and NaCI control.
[c] P < 0.001, EV control versus oil control and NaCI control. For obvious reasons it is not possible to subject control animals to true sham needle insertion. As soon as a needle penetrates the skin, it may be seen as a form of sensory stimulation that activates afferent nerve fibers. If a sham needle insertion without electrical stimulation is performed, then different acupuncture methods/stimulation techniques are being compared, and this does not provide proper information on the effect of EA versus no EA. We chose EA because the stimulation intensity is easy to standardize and it has been shown to be superior to manual needle stimulation [37]. In addition, to show a difference between two or more stimulation techniques would require a very large number of study subjects. In the present study, the control rats received the same enfluran anesthesia protocol as the rats treated with EA, which, in our opinion, is the best way to control completely environmental and/or emotional factors and the EA effect. The acupuncture needles in the present study were placed in the somatic segments that correspond to ovarian innervation. The needles were stimulated with low frequency EA for optimal activation of muscle nerve afferents to inhibit the autonomic outflow at the segmental level and at the central level and to modulate the HPO axis. The choice of acupuncture points and the aim of stimulation has been the same as in our other EA studies on the female reproductive tract that dealt with blood flow in the uterine arteries prior to in vitro fertilization (IVF) [38], pain-relief during oocyte aspiration in connection with IVF treatment [39], and induction of ovulation in women with PCOS [3]. We have shown that repeated EA treatments restore regular ovulations in more than one-third of the anovulatory women with PCOS. In addition, EA-influenced neuroendocrine and endocrine parameters indicative of PCOS, such as LH/FSH ratios, mean testosterone concentrations, and ß-endorphin concentrations, decreased significantiy [3]. The effects of repeated EA on anovulation were then attributed to an inhibition of hyperactivity in the sympathetic nervous system [3, 5, 6].
The findings of the present study support recent reports that ovarian NGF concentrations in rats with experimentally induced PCO [11] are elevated and that this increase can be related to a hyperactivity in the ovarian sympathetic nerves. Lara et al. [11] also suggests that activation of this neurotrophic-neurogenic regulatory loop is a component of the pathological process by which EV induces cyst formation and anovulation. They also stated that there is evidence that the alteration in neurotrophic input to the ovary contributes to the etiology and/or maintenance of human PCOS [11]. Furthermore, the present study shows that repeated EA treatments reduce peripheral sympathetic nerve hyperactivity, as revealed by the reduction in increased NGF concentrations in the ovaries into a normal range 30 days after EV injection, that did not differ from that of the untreated oil and NaCI control groups. It remains to be shown whether EA directly affects sympathetic nerve activity. Measurements of the nervous output by analyses of the catecholamine release can resolve this. In addition, because receptors for NGF are expressed on the endocrine cells of the ovary, activities of ovarian NGF may mediate and/or be mediated by alterations in endocrine factors, for example, by corticotropin-releasing hormone, prolactin, oxytocin, and/or adrenal corticosteroid secretion. To resolve this, the same experimental protocol regarding EA and controls used here must be supplemented with measurements of serum levels of these hormones. Whether this condition can be reversed with EA treatment at higher stimulation intensities, in higher numbers, and/or over longer periods remains to be shown. The conclusion of this study is that repeated EA treatments reduce ovarian NGF concentrations to within normal ranges. This suggests that EA inhibits the hyperactivity in the ovarian sympathetic nerves, which may be of importance for the development and maintenance of experimentally induced PCO.
ACKNOWLEDGMENTS
The authors thank Professor Owe Lundgren and laboratory assistant Britt-Marie Fin, Department of Physiology, Goteborg University, for providing excellent working facilities and for invaluable laboratory help at their Department. We also thank Associate Professor Folke Knutsson for his invaluable assistance in the morphological analyses of the ovaries. Carl Lofman, M.D., Stockholm is acknowledged for skillful preparation of morphological specimens.
REFERENCES
- Franks S. Polycystic ovary syndrome Arch Dis Child 1997; 77:89-90.
- Dahlgren E, Janson PO, Johansson S, Lapidus L, Lindstedt G, Tengborn L. Hemostatic and metabolic variables in women with polycystic ovary syndrome. Fertil Steril 1994; 61:455-460.
- Stener-Victorin E, Waldenstrom U, Tagnfors U, Lundeberg T, Lindstedt G, Janson PO. Effects of electro-acupuncture on anovulation in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand 2000; 79:180-188.
- Gerhard I, Postneek F. Auricular acupuncture in the treatment of female infertility. Gynecol Endocrinol 1992; 6:171-181.
- Chen BY, Yu J. Relationship between blood radioimmunoreactive beta-endorphin and hand skin temperature during the electro-acupuncture induction of ovulation. Acupunct Electro-Ther Res 1991; 16:1-5.
- Chen BY. Acupuncture normalizes dysfunction of hypothalamic-pituitary-ovarian axis. Acupunct Electro-Ther Res 1997: 22:97-108.
- Brawer JR, Naftolin F, Martin J, Sonnenschein C. Effects of a single injection of estradiol valerate on the hypothalamic arcuate nucleus and on reproductive function in the female rat. Endocrinology 1978; 103:501-512.
- Brawer JR, Munoz M, Farookhi R. Development of the polycystic ovarian condition (PCO) in the estradiol valerate-treated rat. Biol Reprod 1986; 35:647-655.
- Barria A, Leyton V, Ojeda SR, Lara HE. Ovarian steroidal response to gonadotropins and beta-adrenergic stimulation is enhanced in polycyslic ovary syndrome: role of sympathetic innervation. Endocrinology 1993; 133:2696-2703.
- Lara HE, Ferruz JL, Luza S, Bustamante DA, Borges Y, Ojeda SR. Activation of ovarian sympathetic nerves in polycistic ovary syndrome. Endocrinology 1993; 133:2690-2695.
- Lara HE, Dissen GA, Leylon V, Paredes A, Fuenzalida H, Fedler JL, Ojeda SR. An increased intraovarian synthesis of nerve growth factor and its low affinity receptor is a principal component of steroid-induced polycystic ovary in the rat. Endocrinology 2000; 141:1059-1072.
- Lobo RA, Granger LR, Paul WL, Goebelsmann U, Mishell DR Jr. Psychological stress and increases in urinay norepinephrine metabolites, platelet serotonin. and adrenal androgens in women with polycystic ovary syndrome. Am J Obstet Gynecol 1983; 115:496-503.
- Lobo RA. The role of neurotransmitters and opioids in polycystic ovarian syndrome. Endocrinol Metab Clin North Arn 1988; 17:667-683.
- Lara HE, McDonald JK, Ojeda SR. Involvement of nerve growth factor in female sexual development. Endocrinology 1990; 126:364-375.
- Dissen GA, Hill DF, Costa ME, Les Dees CW, Lara HE, Ojeda SR, A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinology 1996: 137:198-209.
- Levi-Montalcini R, Angeleni PU. Immunosympathectomy. Pharmacol Rev 1966; 18:619-628.
- Johnson EMJ, Osbom PA, Rydel RE, Schmidt RE, Pearson J. Characterization of the effects of autoimmune nerve growth factor deprivation in the developing guinea pig. Neuroscience 1983; 8:631-642.
- Aloe L. The effect of nerve growth factor and its antibody on mast cells in vivo. J Neuroimmunol 1988; 18:1-12.
- Lockhart ST, Turrigiano GG, Birren SJ. Nerve growth factor modulates synaptic transmission between sympathetic neurons and cardiac myocytes. J Neurosci 1997; 17:9573-9582.
- Cowen T, Gavazzi I. Plasticity in adult and ageing sympathetic neurons. Prog Neurobiol 1998; 54:249-288.
- Heath BM, Xia J, Dong E, An RH, Brooks A, Liang C, Federoff HG, Kass RS. Overexpression of nerve growth factor in the heart alters ion channel activity and beta-adrenergic signalling in an adult transgenic mouse. J Physiol (Lond) 1998; 512:779-791.
- Lockhart ST, Mead JN, Pisano JM, Slonimsky JD, Birren SJ. Nerve growth factor collaborates with myocyte-derived factors to promote development of presynaptic sites in cultured sympathetic neurons. J Neurobiol 2000; 42:460-476.
- Andersson S. The functional background in acupuncture effects. Scand J Rehabil Med Suppl 1993; 29:31-60.
- Andersson S, Lundeberg T. Acupuncture--from empiricism to science: functional background to acupuncture effects in pain and disease. Med Hypotheses 1995; 45:271-281.
- Sato A, Sato Y, Schmidt RF. The Impact of Somatosensory Input on Autonomic Functions. Heidelberg: Springer-Verlag; 1997.
- Lundeberg T, Hurtig T, Lundeberg S, Thomas M. Long-term results of acupuncture in chronic head and neck pain. Pain Clinic 1988; 2:161-164.
- Haker E, Lundeberg T. Acupuncture treatment in epicondylalgia: a comparative study of two acupuncture techniques. Clin J Pain 1990; 6:221-226.
- Bocchini V, Angeletti PU. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A 1969; 64:787-794.
- Wescamp G, Otten U. An enzyme-linked immunoassay for nerve growth factor (NGF): a tool for studying regulatory mechanisms involved in NGF production in brain and peripheral tissues. J Neurochem 1987; 48:1779-1786.
- Tirassa P, Stenfors C, Lundeberg T, Aloe L. Cholecystokinin-8 regulation of NGF concentrations in adult mouse brain through a mechanism involving CCKA and CCKB receptors. Br J Pharmacol 1998; 123(6):1230-1236.
- Hirshfield AN. Rescue of atretic follicles in vitro and in vivo. Biol Reprod 1989; 40:181-190.
- Junquiera LC, Cameiro J, Kelly RO. Basic Histology. Norwalk: Appleton and Lange; 1989.
- Bagavandoss P, Midgley ARJ, Wicha M. Developmental changes in the follicular basal lamina detected by immunofluorescence and electron microscopy. J Histochem Cytochem 1983; 31:633-640.
- Hay MF, Cran DG, Moor RM. Structural changes occurring during atresia in sheep ovarian follicles. Cell Tissue Res 1976; 169:515-529.
- Nakamura Y. Treatment of polycystic ovary syndrome: an overview. Horm Res 1990; 33:(suppl 2):31.
- Vaitukaitis JL. Polycystic-ovary syndrome-what is it? N Engl J Med 1983; 309:1245-1246.
- Litscher G, Wang L, Yang NH, Schwarz G. Ultrasound-monitored effects of acupuncture on brain and eye. Neurol Res 1999; 21:373-377.
- Stener Victorin E, Waldenström U, Andersson SA, Wikland M. Reduction of blood flow impedance in the uterine arteries of infertile women with electro-acupuncture. Hum Reprod 1996; 11:1314-1317.
- Stener-Victorin E, Waldenström U, Nilsson L, Wikland M, Janson PO. A prospective randomized study of electro-acupuncture versus alfentanil as anaesthesia during oocyte aspiration in in-vitro fertilization. Hum Reprod 1999; 14:2480-2484.
[1]Supported by grants from the Hjalmar Svensson Foundation, Wilhelm och Martina Lundgrens Vetenskapsfond Wilhelm and Martina Lundgren's Science Fund, and the Foundation for Acupuncture and Alternative Biological Treatment Methods.
[2]Correspondence: Elisabet Stener-Victorin, Department of Obstetrics and Gynecology, Kvinnokliniken, Sahlgrenska University Hospital, SE-413 45 Göteborg, Sweden. FAX: 46 31829248;
e-mail: elisabet.stenervictorin@medstud.gu.se
Received: 29 February 2000.
First decision: 30 March 2000.
Accepted: 11 July 2000.
© 2000 by the Society for the Study of Reproduction, Inc.
ISSN: 0006-3363. http://www.biolreprod.org |





